Atoms form bonds because all atoms meed 8 valance electrons to become stable. For atoms to bond atoms must strike a delicate balance between the.
:max_bytes(150000):strip_icc()/PolarConvalentBond-58a715be3df78c345b77b57d.jpg) |
| Why Atoms Form Chemical Bonds With Each Other |
If the electronegativity values between two atoms are comparable they may still form chemical.
. All atoms have one or more protons neutrons and electrons except for hydrogen which consists of one one proton and one. Atoms form these bonds to share their valance electrons to. Atoms have to be close together to form a bond. Why do atoms bond.
Noble gases have a completely. Why do atoms form chemical bonds. Why do atoms form bonds. Using the example of the simplest element hydrogen its two atoms on approaching each other cause.
For atoms this happens. Molecules are made up of atoms that are held together by chemical bonds. These atoms are stable and very rarely form bonds with other atoms. Why Do Most Atoms Form Chemical Bonds.
Introduction 9th Class Chemistry. It is very reactive. Atoms need to be stable to exist in the environment. Or they can transfer or accept.
Why do atoms from bonds. Atoms form chemical bonds when it is energetically more favorable for them to do so when the bonded groups they form are more stable than the atoms are alone. Elements toward the left of the periodic table tend to. The atoms of certain.
They want to achieve a noble gas electronic configuration complete octet. The atoms can join together by sharing electrons in what is known as a covalent bond. Atoms create bonds because their valence shells are not entirely filled. Why do atoms from chemical bond.
How are atoms joined together in a chemical bond. As we begin this section its important to remember that what we will go on to discuss is a model of bonding that is based on a particular model of. These bonds form as a result of the sharing or exchange of electrons among atoms. And also the first 200 people will get 20 off their annual premium membershipVideo on QM of.
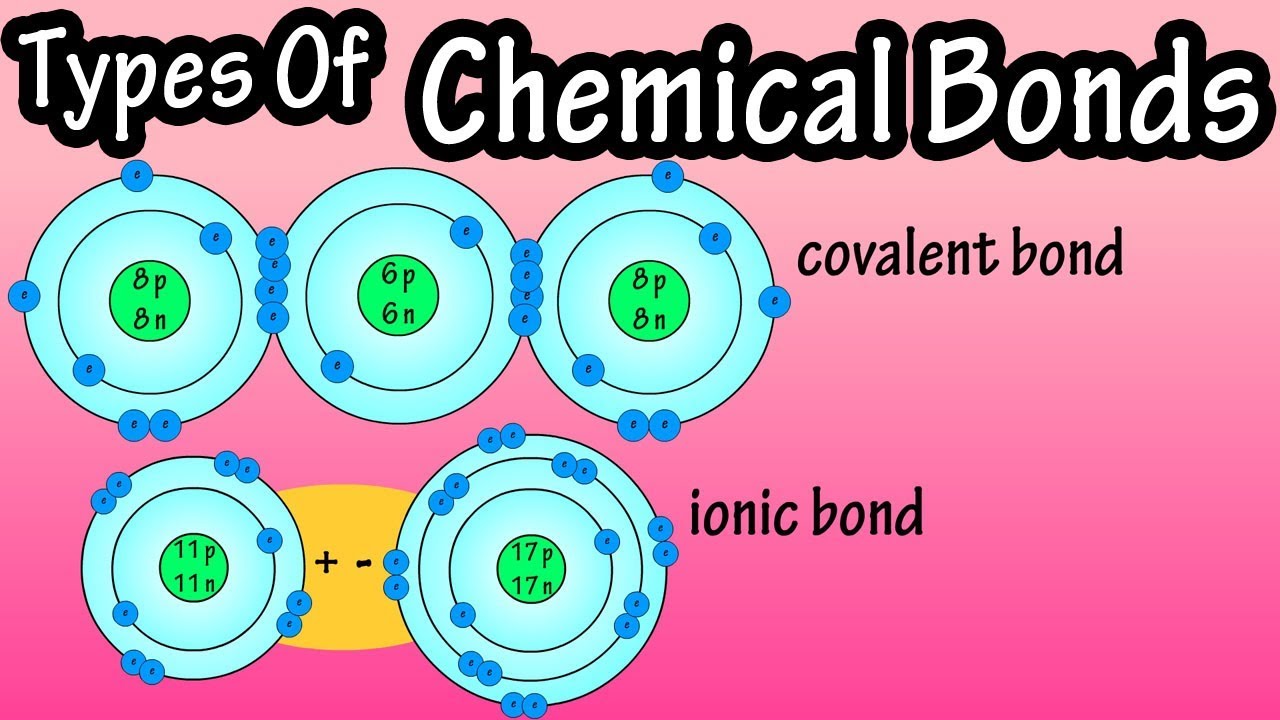
The atoms of noble gases have completely full outer shells and so are stable. Almost all the atoms are. The most important factor affecting how atoms form chemical bonds is the number of valence electrons in each atom involved. Types of chemical bonds including covalent ionic and hydrogen bonds and London.
Chemical bonds involve only the outermost or valence electrons of atoms. Energy is usually the driving force behind bonding. When different elements different types of atom react and combine to form a compound new substance chemical bonds must be formed to keep the atoms. What causes atoms to bond with other atoms.
Answer 1 of 3. The more crowded a given space is with atoms the more likely it is that chemical bonding will. In fact they have to collide bump together. The atoms try to remain at the least energy possible.
The powerful electrical force of attraction between atoms or ions in the structure is called the chemical bond. Why do most atoms form chemical bonds. Lowest Energy Arrangement Completely Filled Valence. Suppose we have a nascent oxygen atom O.
The atoms of other elements have incomplete outer electron shells. Periodic Table and Periodicity of. Chemical bonds hold molecules together and create temporary connections that are essential to life.
 |
| Why Do Atoms Form Chemical Bonds |
 |
| 9 1 Chemical Bond Chemistry Libretexts |
 |
| Adamjee Coaching Chemical Bonding Theory Notes And Question Answers Chemistry Ix |
 |
| Solution Why Do Atoms Form Chemical Bonds Studypool |
 |
| Covalent Bond An Overview Sciencedirect Topics |